Refrigeration
If
you were to double the pressure within a container of air, its
absolute temperature would double.
Since
absolute zero is about -460 degrees F, air starting at 70 degrees
would begin at 70+ 460 = 530 degrees above absolute zero. So, by
doubling the pressure on two cubic feet of 70 degree air (increasing
it to only about 30 PSI), we raise its temperature to 600 degrees F
(530 X 2 - 460 = 600).
Generally
we aren't aware of all this heat, because air stores very little of
it, and so it dissipates almost instantly. However, if you have been
around air compressors, you know they get hot, and now you know why.
When
air expands, it cools according to the same ratios. If you have had
much experience with air-powered tools, you know that frost can form
as the air expands through them. Under these conditions, air is first
compressed, its increase in heat is allowed to escape, and now as it
expands it cools to a lower temperature than that at which it was
stored.
So
why isn't this principle commonly used in refrigeration today? Ask
the refrigeration industry. Theory says it must work, and the frost
on my air-powered grinder says it does work. Furthermore, this
process is often used commercially to achieve cryogenic temperatures.
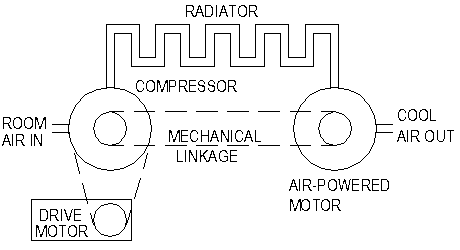
Consider
the drawing below for a practical air-based refrigerator system:
1. Ambient
air is compressed on the left.
2. The
resulting heat is dumped through the radiator.
3. The
air that has been cooled by the radiator is allowed to expand through
an air-powered motor on the right.
4. This
expansion reduces the temperature further – to below the
temperature of the input air.
The
mechanical energy from the air motor is returned to assist the drive
motor in powering the compressor. There would be less energy
returned of course, because the volume of the cooler air at the
right end of the radiator would be less than that of the hot air
entering the left end of the radiator (although obviously they would
be at the same pressure).
For
mechanical simplicity, the entire system (except the radiator) could
be combined on a single shaft as shown below:
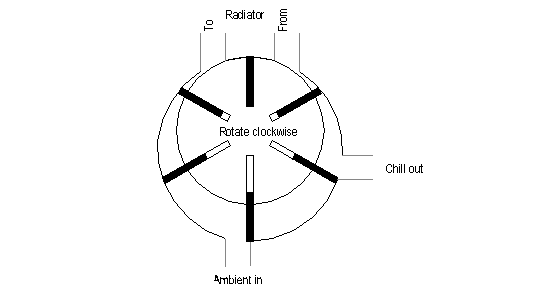 Take another look at this
illustration:
Take another look at this
illustration:
· By
applying pressure to the “to” side of the radiator port, it
becomes an air or steam-driven motor, turning counter-clockwise.
· By
switching the pressure to “from” port, you could drive it
clockwise, albeit at a slightly reduced efficiency.
· By
placing the “in” and “out” ports outside a space to be
heated, and the radiator on the inside, and applying power to rotate
it clockwise, the unit becomes a heat pump.
Radiation
Refrigerator
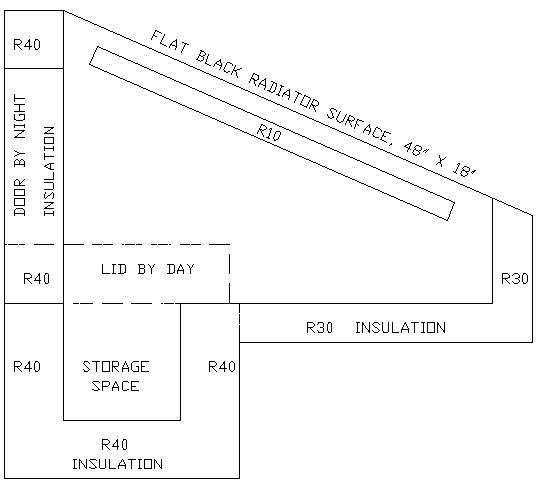
This
idea is based upon the fact that on a clear night, about 20 to 40 BTU
per hour can be radiated into deep space for every square foot of
flat-black surface.
The
design goal (untried) here is to keep two gallons of water or milk
below 45 degrees on a 100 degree day, in a box 12"x 18" x
12" deep.
If
we allowed 2 gallons of water (or milk) to rise in temperature from
32 degrees F to 45 degrees F, this would mean we were losing 217 BTU
(2 X 8.345lbs X 13 degrees).
With
8 square feet of surface area on the box, we would be obtaining
27.125
BTU per square foot (217/8=27.125).
If
we defined the non-cooling portion of the day as 16 hours, we would
be warming the stored fluid by 27.125/16 = about 1.7 BTU per square
foot per hour.
If
we had an average daytime outside temperature of 100 degrees, the
temperature difference would be 100-32 = 68 degrees. If we divide
this by 1.7 BTU per hour, our insulation would need an "R"
value of 68 / 1.7 = R40.
Counting
on at least 2 hours of 20 BTU per square foot per hour radiation per
night, the radiator surface area we would need to dump 217 BTU during
this time would be: 217 BTU/2 hours/20 BTU per hour = 5.4 square
feet.
Now
this is a lot larger than the top our 12" x 18" cold
storage box, so a cross-section of the radiation refrigerator that I
haven't built yet, would look something like this:
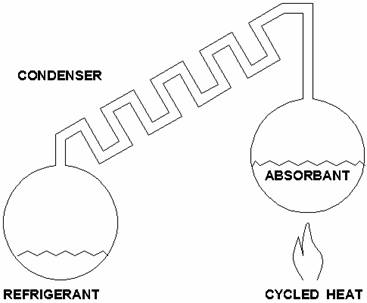
Sounds
like an oxymoron – but wait! Some of you are familiar with
propane-powered refrigerators common in motor homes and camp
trailers. Does it make any sense to get cooling out of a flame?
There’s a trick to it; it’s called absorption cooling.
A
refrigerant is absorbed into a liquid or solid at normal
temperatures, but driven out when the temperature is raised beyond a
certain point. This vapor then passes through a tube to a cooler
place where it is condensed and stored in another container in liquid
form.
When
the heat is off, the material in the original chamber begins to
reabsorb refrigerant vapor in the system and a vacuum is created.
This
causes the refrigerant to draw heat from (cool) its environment as it
evaporates, while being reabsorbed in the original chamber.
As
a residential solar cooler, consider an absorbent system heated by
concentrating the morning sun on the east side of the house. This
drives the refrigerant through the condenser where the heat is
exhausted out of the dwelling, and into the other container in liquid
form.
By
the afternoon, the sun has moved on and the reabsorbing process
begins, cooling the dwelling. It might well be practical to use the
condenser to also provide the cooling surface, by re-routing the air
downward into the dwelling. Conceivably the entire process could be
passive if the condenser were above the dwelling. This is because the
heat and cold would naturally flow upward and downward, respectively.
Stirling
In
the “Engine” category you will find a remarkable device called a
“Stirling Engine”. As an engine it produces mechanical energy if
you have sources of hot and cold, but it has another trick. If you
mechanically rotate it, it will actually produce a temperature
difference. In fact, specially-built Stirling engines are used to
achieve cryogenic (super cold) temperatures.

 Take another look at this
illustration:
Take another look at this
illustration: